Electroflocculation
Wastewater Treatment by Electroflocculation
Published in Chemistry and Material Science Encyclopaedia of Applied Electrochemistry by Dr. Vivian Robinson.
Introduction
Electroflocculation is a process wherein flocculating metal ions are electrolytically added to polluted water at an anode, and gas micro bubbles are released at a cathode. The flocculating metal ions adhere to pollutants in the water, increasing their size, and the gas micro bubbles capture the flocculated pollutants and float them to the surface, from where they can be easily removed. By the appropriate choice of electrode materials, this process can remove a wide variety of pollutants without the need for chemicals or filters, with pH adjustment to near neutral at discharge excluded.
The Process
The Electrochemical Reactions
The main electrochemical reactions involved are:
(1) Anode 1 Al – 3e− → Al3+
(2) Anode 2 Fe – 3e− → Fe3+
(3) Cathode 2H2O + 2e− → 2(OH)−+H2
Both the Al and Fe trivalent ions form as proton donors Al(H2O)63+ and Fe(H2O)63+, which are only stable at low pH [1-3]. At higher pH, they lose protons to form the monomers
Al(H2O)5(OH)2+, Al(H2O)4(OH)2+, Al(OH)3 and Al(OH)4− progressively as the pH increases. Some of these monomers are unstable and try to form the OH bridged dimer Al(H2O)4(OH)2(H2O)4Al4+. These unstable monomers react best at a pH in the range of 5 < pH < 7. This process goes much faster when there are pollutants in the water onto which the dimer can easily form, binding to the pollutant at the same time. The Fe3+ reactions are similar. These are the standard chemical trivalent ion flocculation processes used in water treated by both chemical and electrocoagulation processes.
By supplying the ions electrolytically, the cathodic reaction (3) generates hydrogen gas micro bubbles, which capture the flocculated pollutants and float them to the surface. Under the right conditions, this combination of processes captures the pollutants as a stable surface floc layer that is easily separated from the treated water. Reaction (3) also means that the water’s pH will increase with greater treatment dosing, and its pH must be pre- adjusted such that it is always near neutral at discharge.
The flocculation reactions described above attach Fe or Al oxides to the pollutants. At near neutral pH, these oxides have very low solubility product constants [4,5]. This binding to insoluble oxides effectively makes most captured pollutants non-leachable and suitable for landfill. It has been noted that many pollutants captured in this manner also formed acid resistant compounds that passed TCLP toxicity leach tests [6].
Engineering Requirements
For the process to occur, electrodes of the appropriate material must be placed in water with an electric potential applied across the electrodes to cause an electric current to flow between them. The electrochemical reactions occur instantly upon the passage of electric current. Typically, the flocculating process, whereby the monomers form the bridged dimers, requires many hours to go to completion. This is often referred to as the “incubation” time. In operation, a convenient time must be established between the ultimate pollution removal and the practical completion required for economic operation to the required standard.
For the process to work properly, the water needs to be treated using an anode current density less than 100 A/sq m, with equal cathode area. Above 100 A/sq m, the micro bubbles become too large to produce a stable floc. With “typical” treatment being 100 amp hours per kL for moderately polluted water, that requires at least 1 sq m each of anode and cathode per kL and a current of 100 Amps for a treatment time of 1 hour, or 10 sq m of anode/cathode for a treatment time of 6 minutes. These large electrode surface areas mean that low voltages can pass the required current and that large areas of metal are used in electrode fabrication. The two beneficial effects of this are that the voltage required to power the electrochemical reaction are low, typically 2 – 4 volts, hence power consumption is also low, and electrode replacement is an infrequent occurrence, typically several months to yearly intervals. Electrolytic dosing times of less than a few minutes usually imply too great a current density for stable surface floc formation and hence easy pollutant removal.
The water’s electrical conductivity has a major impact upon power requirements and process time. The lower the conductivity, the greater the voltage and hence power required, or the longer time needed to process it. Otherwise, conductivity has no detected effect upon the treated water quality, even with salinities over ten times greater than that of seawater. The pollutant type also affects the required treatment dose. Larger pollutants, such as clay, require less treatment per unit weight of pollutant than do smaller pollutants, such as starch. For a given pollutant type, the treatment required is linearly proportional to the amount of pollutant to be removed.
When applied correctly, the result is that the pollutants are captured by the trivalent metal ions, most of which float to the surface, with the heaviest sinking to the bottom of the reaction vessel. Those that float to the surface form a stable floc, which can be easily removed. This enables the cleaned water to flow out of the system without the need for other separation techniques, such as filters. In this manner, electroflocculation is like a chemical-free dissolved air flotation (DAF) system or a membrane-free filter. As a filter, it can clean to nanofiltration level, handling some pollutant loadings well over 20,000 mg/L without clogging.
Practical Implementation
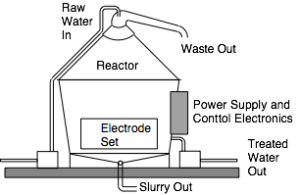
There are two separate mechanisms for treating water by electroflocculation: batch and continuous flow. Both systems involve a reaction chamber into which is placed a set of electrodes. In a batch system, a single chamber holds all the electrodes and water, as illustrated in Figure 1. The water is pumped into the reactor, the current for the electrochemical reactions is passed and the pollutants float to the surface. In this situation, they are best removed by raising the water level, which forces the floc out the top chute. The water rests in the chamber for a pre-determined time before it is pumped out. With batch processes requiring time for the water to be pumped in an out, there is a practical volume limit of about 10 kL per batch, beyond which pump in/out times may be too long to be practical.
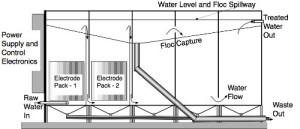
Continuous flow uses multiple chambers, as illustrated in Figure 2. The first two chambers each contain the electrode sets, and the remainder are rest chambers. The water is pumped into the first chamber where one electrochemical reaction occurs, flowing out the top into the bottom of the second chamber. There, the second electrochemical reaction occurs, again generating surface floc, before the water exits again at the top. From there it moves under laminar flow through the rest chambers. The low current density and laminar flow ensure that the captured pollutants form a stable floc layer on the surface, from where it is easily removed by simple mechanical means. Different numbers of electrode sets and/or chambers can be used for different applications, depending upon the nature of the pollutant and the desired result.
Continuous flow electroflocculation can be scaled to any desired size. For practical limitations, a 40,000 liter system that can typically clean 1 mL of water per day could be transported in a 40 ft shipping container. Larger volumes are handled by multiple systems or large plants built on site.
Electroflocculation water treatment systems need to be automated with microprocessor control. This is required to make sure that changing conditions do not prevent successful operation. It is also required to adequately remove the pollutants, including those that may settle to the bottom of the chambers. With appropriate microprocessor control, the one set of hardware can handle a wide variety of pollutants, with different pollutants, pollution levels and desired outcomes being handled by different electronic settings. In practice, if the systems are treating water of uniform quality, they operate for months at a time with only minor maintenance between the electrode changes that are required as a result of reactions (1) and (2) eroding the anodes until there is no longer sufficient metal left to pass current. When raw water quality varies significantly, operator input to adjust the treatment settings is recommended for efficient operation. This could be automated by the use of adequate input water monitoring techniques.
Note that typically about 5% of the water being treated is removed when the wastes are removed from the electroflocculation facility. With the appropriate post-treatment collection, it is possible to separate the pollutants from the water and return the removed water for further processing if required. For example, emulsified fats, oils and greases are liberated and float to the surface. They can be skimmed off and sent for recycling. The dried weight of other removed pollutants is typically the dried weight of the pollutant plus an additional 1% to 10% due to the added metal oxide. This sinks to the bottom when removed from the electroflocculation facility and the micro bubbles broken. The pollutants can be separated by appropriate techniques and, in most cases, the waste can be dried and sent to a landfill as a non-leachable product. In this manner, no liquid waste need be removed from the site. In many situations, the treated water is either recycled or reused. When recycling, losses due to evaporation are replaced by fresh water, keeping salinity build-up to a minimum.
Results and Environmental Benefits
The following is a list of some contaminants that have been removed by electroflocculation [7].
- Suspended solids down to the size of large dissolved molecules, including clay, asbestos and starch
- Organic molecules with a molecular weight greater than ≈ 300 gm/mol
- Free and emulsified fats, oils, greases and hydrocarbons, including benzene ring structures and many halogenated hydrocarbons
- “Sticky” substances, such as glues, polymers and monomers
- Cations with Z > 23 and some lower Z, such as Mg and Ca
- Anions with Z > 33 and some with lower Z, such as F Chemicals such as cyanide, arsenate, and phosphate
- Pathogens such as algae, bacteria and viruses
BOD/COD - Many dye molecules, tannins and humic substances
- Soaps and detergents (MBAS)
It should be noted that removal rates are typically > 99% for most pollutants, a figure that requires sufficient treatment dosage being applied, with the treated water resting for a sufficient time. Many BOD and COD pollutants that consist of small organic molecules of molecular weight less than 150 gm/Mol, such as C2H5OH, CH3COOH and similar, are difficult to remove by this process. This broad array of pollutants covers many of those of interest in the treatment of wastewater. In removing these pollutants, electroflocculation liberates emulsified FOGs, separates most suspended solids and captures the dissolved molecules by forming insoluble metal oxide bonds with them. Low voltages for the electrochemical reactions and low pressure pumping means that electricity consumption is minimal.
These features give electroflocculation a number of environmental advantages. It has found many applications in the food processing industry, removing FOGs, suspended solids and BOD. It has also shown itself to be an ideal pre-treatment for reverse osmosis (RO), almost irrespective of the original water source. In many mining applications, water salinity prevents wastewater disposal to the environment. Desalination by RO is expensive because pollutants often clog the RO membranes. Electroflocculation removes those pollutants that cause problems for RO membranes, making RO desalination a more viable proposition economically.
Future Directions
A big advantage of electroflocculation is its ability to float pollutants to the surface as an insoluble compound between the soluble pollutant and the flocculating Fe and Al metal ions. This offers a unique opportunity for reducing the pollution in many existing large water bodies, such as dams and lakes. Appropriately designed electroflocculation systems could be floated over parts of the water body, powered by either solar and/or DC wind generators if required. The low voltages required means minimum power consumption for maximum pollutant removal. Whenever power is activated, the electroflocculation reaction captures the pollutants and floats them to the surface. Note that the rising micro bubbles draws in and circulates water from great distances away from the electrode sets. At the surface, they could be either constrained or removed from the water, to be dried and sent to a landfill, or simply be allowed to spread over the surface, where they will sink to the bottom when the bubbles eventually burst. There they will form an insoluble precipitate that will not leach into the water and will always settle at the bottom, even when stirred up (it is recommended that radioactive wastes, oily contaminants and highly toxic pollutants be removed rather than retained in the water body.)
In this manner, electroflocculation offers an excellent opportunity for environmental restoration by in situ cleaning of large bodies of water without the need to add chemicals or the need to treat and discharge the water. The only byproducts will be inert common aluminum and iron oxides that are chemically bound in an insoluble bond to the pollutants that have been either removed or settled to the bottom. With the appropriately designed equipment placed in the large water body, all that would be noticed is that the water slowly became clean and restored to a usable condition.
Note that the minor anode reaction 2H2O – 4e− → 4H+ + O2 oxygenates the water, making it a more suitable environment for aquatic life to flourish. The residual Al level is the saturated Al level at the water’s pH, which if near neutral is very low [4,5]. Provided the water is not over treated too much, residual Al levels remain low, avoiding Al toxicity issues.
Cross-References
Electrocoagulation, Electroflotation
References
1. Phillips CSG, Williams RJP (1966) Inorganic Chemistry, Vol 1, Oxford University Press, Oxford, Ch 14
2. Phillips CSG, Williams RJP (1966) Inorganic Chemistry, Vol 2, Oxford University Press, Oxford, p 524
3. Morrison TI, Reis, AH, Knapp GS, Fradin FY, Chen H, Klippert E (1978) J. Am. Chem. Soc., 100, p 3326
4. Baes CF Jr, Mesmer RE (1976) The Hydrolysis of Cations, Wiley Interscience, NY, Ch 6
5. Lide R (Ed) (1999 ) CRC Handbook of Chemistry and Physics, The Chemical Rubber Co. (http://www.ktf-split.hr/periodni/en/abc/kpt.html)
6. US EPA Report No. EPA/540/R-96/502 (1998)
7. Robinson VNE (1999) AWWA Regional Conference, Albury, NSW p 181